Cat: HF-1001A
Cat: HF-1001A
IL1-alpha, Human, HEK293 Cells,Tag Free: Product Information
Hematopoietin-1; IL1 alpha; IL-1 alpha; IL1; IL1A; IL-1A; IL1-ALPHA; IL1F1; IL-1F1;BAF
Q53QF9
Human embryonic kidney cell, HEK293-derived human IL-1 alpha/IL-1F1 protein
Ser113-Ala271
18.0 kDa
Solution protein
Dissolved in sterile PBS buffer.
This solution can be diluted into other aqueous buffers. Centrifuge the vial prior to opening.
Avoid repeated freeze-thaw cycles. It is recommended that the protein be aliquoted for optimal storage. 12 months from date of receipt, -20 to -70 °C as supplied.
Shipping with dry ice
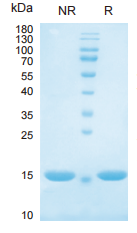
> 95%, determined by SDS-PAGE.
<0.010 EU per 1 ug of the protein by the LAL method.
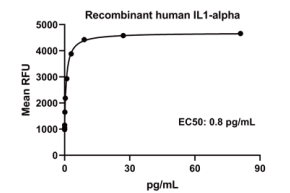
Measured in a cell proliferation assay using D10.G4.1 mouse helper T cells. The EC50 for this effect is 0.6-6 pg/mL.
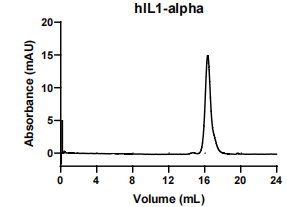
IL1-alpha, Human, HEK293 Cells,Tag Free:SDS-PAGE & Bioactivity
IL1-alpha, Human, HEK293 Cells,Tag Free:Synonyms
Hematopoietin-1; IL1 alpha; IL-1 alpha; IL1; IL1A; IL-1A; IL1-ALPHA; IL1F1; IL-1F1;BAF
IL1-alpha, Human, HEK293 Cells,Tag Free:Background
Interleukin 1 (IL-1) , is a name that designates two proteins, IL-1 alpha and IL-1 beta, which are the products of distinct genes, but which show approximately 25% amino acid sequence identity and which recognize the same cell surface receptors. Although IL-1 production is generally considered to be a consequence of inflammation, recent evidence suggests that IL-1 is also temporarily upregulated during bone formation and the menstrual cycle and can be induced in response to nervous system stimulation. In response to classic stimuli produced by inflammatory agents, infections or microbial endotoxins, a dramatic increase in the production of IL-1 by macrophages and various other cells is seen. Cells in particular known to produce IL-1 include osteoblasts, monocytes, macrophages, keratinocytes, Kupffer cells, hepatocytes, thymic and salivary gland epithelium, Schwann cells, fibroblasts and glia (oligodendroglia, astrocytes and microglia). IL-1 alpha and IL-1 beta are both synthesized as 31 kDa precursors that are subsequently cleaved into proteins with molecular weights of approximately 17,000 Da. Neither precursor contains a typical hydrophobic signal peptide sequence and most of the precursor form of IL-1 alpha remains in the cytosol of cells, although there is evidence for a membrane-bound form of the precursor form of IL-1 alpha. The IL-1 alpha precursor reportedly shows full biological activity in the EL-4 assay. Among various species, the amino acid sequence of mature IL-1 alpha is conserved 60% to 70% and human IL-1 has been found to be biologically active on murine cell lines. Both forms of IL-1 bind to the same receptors, designated type I and type II. Evidence suggests that only the type I receptor is capable of signal transduction and that the type II receptor may function as a decoy, binding IL-1 and thus preventing binding of IL-1 to the type I receptor.
1. Nicklin MJ,et al. (1994) Genomics. 19(2):382-4.
2. March CJ, et al. (1985) Nature. 315(6021):641-7.
3. Bankers-Fulbright JL, et al. (1996) Life Sci. 59(2):61-83.
4. Dinarello CA, et al. (1997) Semin Oncol. 24 (3 Suppl 9):S9-81-S9-93.