Cat: HF-2030
Cat: HF-2030
IFNB, Human, HEK293 Cells,Tag Free: Product Information
P01574
Human embryonic kidney cell, HEK293-derived human IFN-beta protein
Met22-Asn187
20.0 kDa
Solution protein.
Dissolved in sterile PBS buffer. This solution can be diluted into other aqueous buffers. Centrifuge the vial prior to opening.
Avoid repeated freeze-thaw cycles. It is recommended that the protein be aliquoted for optimal storage. 12 months from date of receipt, -20 to -70 °C as supplied.
Shipping with dry ice
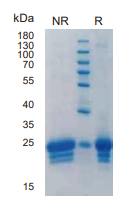
> 95%, determined by SDS-PAGE
<0.010 EU per 1 ug of the protein by the LAL method
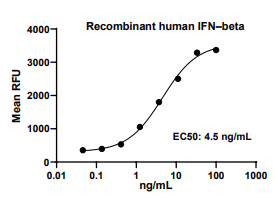
Measured in anti-viral assays using HeLa human cervical epithelial carcinoma cells infected with encephalomyocarditis (EMC) virus.The EC50 for this effect is 1-10 ng/mL.
IFNB, Human, HEK293 Cells,Tag Free:SDS-PAGE & Bioactivity
IFNB, Human, HEK293 Cells,Tag Free:Synonyms
Fibroblast interferon; IFB; IFBIFNB; IFF; IFNB; IFNB1; IFNbeta; IFN-beta; interferon beta
IFNB, Human, HEK293 Cells,Tag Free:Background
Interferon beta (IFN-beta ) , also known as fibroblast IFN, is a secreted, approximately 22 kDa member of the type I interferon family of molecules (1). Mature human IFN-beta shares 47% and 46% amino acid sequence identity with the mouse and rat proteins, respectively. Fibroblasts are the major
producers of IFN-beta, but it can also be produced by dendritic cells, macrophages, and endothelial cells in response to pathogen exposure (2). It is trans-criptionally regulated by TRAF3, IRF3, IRF7, and NF-kappa B (3). Following secretion, IFN-beta signals through the heterodimeric IFN-alpha / beta
Receptor and activates the JAK/STAT signaling pathway (4-7). IFN-beta -deficient mice show increased susceptibility to experimental autoimmune
encephalomyelitis (EAE), a disease model of human multiple sclerosis (MS) (8). Furthermore, IFN-beta has been shown to suppress the Th17 cell response in both MS and EAE and has commonly been used as a treatment for MS (9-13).
1. González-Navajas, J.M. et al. (2012) Nat. Rev. Immunol. 12:125.
2. Reder, A.T. and X. Feng (2013) Front. Immunol. 4:281.
3. Schafer, S.L. et al. (1998) J. Biol. Chem. 273:2714.
4. Honda, K. et al. (2006) Nat. Rev. Immunol. 6:644.
5. Platanias, L.C. (2005) Nat. Rev. Immunol. 5:375.
6. Fasler-Kan, E. et al. (1998) Eur. J. Biochem. 254:514.
7. Matikainen, S. et al. (1999) Blood 93:1980.
8. Teige, I. et al. (2003) J. Immunol. 170:4776.
9. Shinohara, M.L. et al. (2008) Immunity 29:68.
10.Guo, B. et al. (2008) J. Clin. Invest. 118:1680.
11.Ramgolam, V.S. and S. Markovic-Plese (2010) Endocr. Metab. Immune Disord. Drug Targets 10:161.
12. Martín-Saavedra, F.M. et al. (2008) Mol. Immunol. 45:4008.
13. Inoue M. and M.L. Shinohara (2013) Immunology 139:11.