Cat: HF-2003
Cat: HF-2003
M-CSF/CSF1, Human, HEK293 Cells,Tag Free: Product Information
P09603
Human embryonic kidney cell, HEK293-derived human M-CSF/CSF1 protein
Glu33-Ser190
22.0 kDa(Monomer)
Dimer in solution
Solution protein.
Dissolved in sterile PBS buffer. This solution can be diluted into other aqueous buffers. Centrifuge the vial prior to opening.
Avoid repeated freeze-thaw cycles. It is recommended that the protein be aliquoted for optimal storage. 12 months from date of receipt, -20 to -70 °C as supplied.
Shipping with dry ice
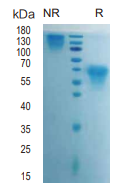
> 95%, determined by SDS-PAGE
<0.010 EU per 1 ug of the protein by the LAL method
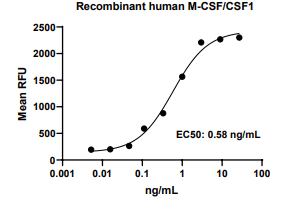
Measured in a cell proliferation assay using M-NFS-60 mouse myelogenous leukemia lymphoblast cells. The EC50 for this effect is 0.2-1.5 ng/mL.
M-CSF/CSF1, Human, HEK293 Cells,Tag Free:SDS-PAGE & Bioactivity
M-CSF/CSF1, Human, HEK293 Cells,Tag Free:Synonyms
Colony stimulating factor 1 (macrophage); CSF1; CSF-1; macrophage colony-stimulating factor 1; MCSF
M-CSF/CSF1, Human, HEK293 Cells,Tag Free:Background
Macrophage Colony Stimulating Factor(M-CSF), also known as CSF-1, is a four-alpha -helical-bundle cytokine that is the primary regulator of macrophage survival, proliferation and differentiation (1-3). M-CSF is also essential for the survival and proliferation of osteoclast progenitors (1, 4). M-CSF also primes and enhances macrophage killing of tumor cells and microorganisms, regulates the release of cytokines and other inflammatory
modulators from macrophages, and stimulates pinocytosis (2, 3). M-CSF increases during pregnancy to support implantation and growth of the decidua and placenta (5). Sources of M-CSF include fibroblasts, activated macrophages, endometrial secretory epithelium, bone marrow stromal cells and
activated endothelial cells (1-5). The M-CSF receptor (c-fms) transduces its pleotropic effects and mediates its endocytosis. M-CSF mRNAs of varioussizes occur (3-9). Full length human M-CSF transcripts encode a 522 amino acid (aa) type I transmembrane (TM) protein with a 464 aa extracellular
region, a 21 aa TM domain, and a 37 aa cytoplasmic tail that forms a 140 kDa covalent dimer. Differential processing produces two proteolytically
cleaved, secreted dimers. One is an N- and O- glycosylated 86 kDa dimer, while the other is modified by both glycosylation and chondroitin-sulfate
proteoglycan (PG) to generate a 200 kDa subunit. Although PG-modified M-CSF can circulate, it may be immobilized by attachment to type V collagen
(8). Shorter transcripts encode M-CSF that lacks cleavage and PG sites and produces an N-glycosylated 68 kDa TM dimer and a slowly produced 44
kDa secreted dimer (7). Although forms may vary in activity and half-life, all contain the N-terminal 150 aa portion (10).
1. Pixley, F.J. and E.R. Stanley (2004) Trends Cell Biol. 14:628.
2.Chitu, V. and E.R. Stanley (2006) Curr. Opin. Immunol. 18:39.
3. Fixe, P. and V. Praloran (1997) Eur. Cytokine Netw. 8:125.
4. Ryan, G.R. et al. (2001) Blood 98:74.
5. Makrigiannakis, A. et al. (2006) Trends Endocrinol. Metab. 17:178.
6. Nandi, S. et al. (2006) Blood 107:786.
7. Rettenmier, C.W. and M.F. Roussel (1988) Mol. Cell Biol. 8:5026.
8. Suzu, S. et al. (1992) J. Biol. Chem. 267:16812.
9. Manos, M.M. (1988) Mol. Cell. Biol. 8:5035.
10. Koths, K. (1997) Mol. Reprod. Dev. 46:31.