Cat: HF-2064
IGFBP3, Human, HEK293 Cells,Tag Free: Product Information
P17936
Human embryonic kidney cell, HEK293-derived human IGFBP3 protein
Gly28-Lys291
28.7 kDa
Solution protein.
Dissolved in sterile PBS buffer .
This solution can be diluted into other aqueous buffers. Centrifuge the vial prior to opening.
Avoid repeated freeze-thaw cycles. It is recommended that the protein be aliquoted for optimal storage. 12 months from date of receipt, -20 to -70 °C as supplied.
Shipping with dry ice
> 95%, determined by SDS-PAGE.
<0.010 EU per 1 ug of the protein by the LAL method.
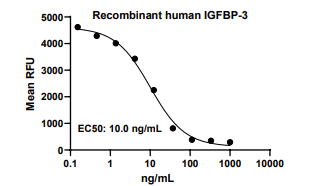
Measured by its ability to inhibit the biological activity of IGF-I or IGF-II on MCF-7 human breast cancer cells. The EC50 for this effect is 6-14 ng/mL.
IGFBP3, Human, HEK293 Cells,Tag Free:SDS-PAGE & Bioactivity
IGFBP3, Human, HEK293 Cells,Tag Free:Synonyms
growth hormone-dependent binding protein; IBP-3; IBP3BP-53; IGF-binding protein 3;IGFBP-3;
IGFBP3, Human, HEK293 Cells,Tag Free:Background
Insulin-like growth factor binding protein-3 (IGFBP-3) is one of six members of the insulinlike growth factor (IGF) binding protein superfamily which function to modulate the biological activity of IGF (1). Human IGFBP-3 is the major binding protein of IGF where it exists in circulation as a ternary complex with the acid-labile subunit (ALS) (2). Like other IGFBP members, human IGFBP-3 includes a cysteine-rich C-terminal domain, a highly
variable central linker domain, and another N-terminal cysteine-rich domain (2, 3). Human IGFBP-3 cDNA encodes a 291 amino acid (aa) precursor
protein with a 27 aa signal peptide that is processed to generate the 264 aa mature protein. Mature human IGFBP-3 shares 82% aa sequence identity
with both mouse and rat IGFBP-3. Post-translational glycosylation and phosphorylation of IGFBP-3 modifies the affinities of the binding protein.
Proteolysis of IGFBP-3 by tissue plasminogen activator (tPA), a disintegrin and metaloproteases (ADAMs), and prostate specific antigen (PSA)
contributes to IGFBP-3 degradation or a reduction in its affinity for IGF (4-6). The majority of soluble IGFBP-3 found in circulation is secreted from
hepatic non-parenchymal cells. IGFBP-3 expression can be modulated by p53 as well as by various cytokines and growth factors (7, 8). In addition to its role in stabilizing and transporting circulating IGF, IGFBP-3 has been shown to potentiate EGF-EGFR-mediated cell growth through the activation of
sphingosine kinase1 (SPHK1) and sphingosin-1-phosphate (S1P) (9, 10). IGFBP-3 has also been shown to modulate adipogenesis (11). Binding of
IGFBP-3 to non-IGF-related ligands has been shown to regulate TGF-beta signaling, DNA damage, apoptosis, autophagy, and gene transcription (12).
1. Shimasaki, S. and N. Ling (1991) Prog. Growth Factor Res. 3:243.
2. Baxter, R.C. (2013) J. Cell Commun. Signal 7:179.
3. Baxter, R.C. (2014) Nat. Rev. Cancer 14:329.
4. Mochizuki, S. et al. (2004) Biochem. Biophys. Res. Commun. 315:79.
5. Cohen, P. et al. (1994) J. Endocrinol. 142:407.
6. Bang, P. (1995) Prog. Growth Factor Res. 6:285.
7. Perks, C.M. and J.M. Holly (2008) J. Mammary Gland Biol. Neoplasia 13:455.
8. Chan, K. and E.M. Spencer (1997) Endocrine 7:95.
9. Guix, M. et al. (2008) J. Clin. Invest. 118:2609.
10. Martin, J.L. et al. (2009) J. Biol. Chem. 284:25542.
11. Chan, S.S. et al. (2009) Am. J. Physiol. Endocrinol. Metab. 296:E654.
12. Martin, J.L. and R.C. Baxter (2011) Growth Factors 29:235