Cat: HF-2035
Cat: HF-2035
SHH, Human, HEK293 Cells,Tag Free: Product Information
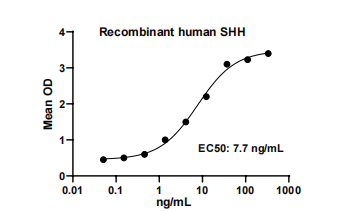
Measured by its ability to induce alkaline phosphatase production by C3H10T1/2 mouse embryonic fibroblast cells. The EC50 for this effect is typically 5-15 ng/mL.
<0.010 EU per 1 ug of the protein by the LAL method.
> 95%, determined by SDS-PAGE.
Shipping with dry ice
Avoid repeated freeze-thaw cycles.
It is recommended that the protein be aliquoted for optimal storage.
12 months from date of receipt, -20 to -70 °C as supplied.
Solution protein
Dissolved in sterile PBS buffer.
This solution can be diluted into other aqueous buffers. Centrifuge the vial prior to opening.
Dimer in solution
19.6 kDa
Human embryonic kidney cell, HEK293-derived human Sonic Hedgehog/Shh protein
Cys24-Gly197
Q15465
Shh;HHG1; HHG-1; HLP3; HPE3; MCOPCB5;Sonic Hedgehog; TPT; TPTPS
SHH, Human, HEK293 Cells,Tag Free:SDS-PAGE & Bioactivity
SHH, Human, HEK293 Cells,Tag Free:Synonyms
Shh;HHG1; HHG-1; HLP3; HPE3; MCOPCB5;Sonic Hedgehog; TPT; TPTPS
SHH, Human, HEK293 Cells,Tag Free:Background
Sonic Hedgehog (Shh) is expressed in embryonic tissues that are critical for the patterning of the developing central nervous system, somite, and limb. It is also involved in whisker, hair, foregut, tooth, and bone development. Shh regulates neural and hematopoietic stem cell fate and is important for thymocyte differentiation and proliferation as well as T cell determination. In adult tissue Shh is associated with cancer development and tissue remodeling following injury (1-3). Human Shh encodes a 462 amino acid (aa) precursor protein that is autocatalytically processed to yield a non-glycosylated 19 kDa N-terminal fragment (Shh-N) and a glycosylated 25 kDa C-terminal protein (Shh-C) (4). Shh-C, which is responsible for the intramolecular processing of Shh, is rapidly degraded following Shh proteolysis (5). Shh-N is highly conserved, sharing >98% aa identity between mouse, human, rat, canine, porcine, and chicken Shh-N. Shh-N can be palmitoylated at its N-terminal cysteine and modified by cholesterol addition at its C-terminus (6). These modifications contribute to the membrane tethering of Shh as well as its assembly into various sized multimers (6-9). Lipid modification and multimerization greatly increase Shh-N receptor binding affinity and signaling potency (5, 6, 8, 9). Monomeric and multimeric Shh can be released from the plasma membrane by the cooperative action of DISP1, SCUBE2, and TACE/ADAM17 (10-12). Modifications also extend the effective range of Shh functionality and are required for the development of protein gradients important in tissue morphogenesis (9, 13). Canonical signaling of Shh is mediated by a multicomponent receptor complex that includes Patched (PTCH1, PTCH2) and Smoothened (SMO) (14)
1. Briscoe, J. and P.P. Therond (2013) Mol. Cell. Biol. 14:416.
2. Aviles, E.C. et al. (2013) Front. Cell. Neurosci. 7:86.
3. Xie, J. et al. (2013) OncoTargets Ther. 6:1425.
4. Marigo, V. et al. (1995) Genomics 28:44.
5. Zeng, X. et al. (2001) Nature 411:716.
6. Feng, J. et al. (2004) Development 131:4357.
7. Goetz, J.A. et al. (2006) J. Biol. Chem. 281:4087.
8. Pepinsky, R.B. et al. (1998) J. Biol. Chem. 273:14037.
9. Pepinsky, R.B. et al. (1998) J. Biol. Chem. 273:14037.
10. Chen, M.-H. et al. (2004) Genes Dev. 18:641.
11. Etheridge, L.A. et al. (2010) Development 137:133.
12. Jakobs, P. et al. (2014) J. Cell Sci. 127:1726.
13. Dierker, T. et al. (2009) J. Biol. Chem. 284:8013.
14. Lewis, P.M. et al. (2001) Cell 105:599