Cat: MF-2008
Cat: MF-2008
EGF, Mouse, HEK293 Cells,Tag Free: Product Information
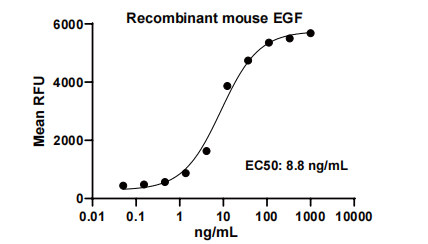
Measured in a cell proliferation assay using Balb/3T3 mouse embryonic fibroblast cells.
The EC50 for this effect is 5-20 ng/mL.
<0.010 EU per 1 ug of the protein by the LAL method
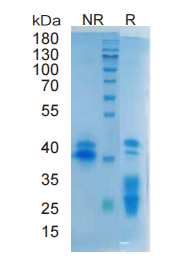
> 95%, determined by SDS-PAGE
Shipping with dry ice
Avoid repeated freeze-thaw cycles.
It is recommended that the protein be aliquoted for optimal storage.
12 months from date of receipt, -20 to -70 °C as supplied.
Solution protein.
Dissolved in sterile PBS buffer.
This solution can be diluted into other aqueous buffers. Centrifuge the vial prior to opening.
6.0 kDa
Human embryonic kidney cell, HEK293-derived mouse EGF protein
Asn977-Arg1029
P01132
beta-urogastrone; EGF; epidermal growth factor (beta-urogastrone); epidermal growth factor; hEGF
EGF, Mouse, HEK293 Cells,Tag Free:SDS-PAGE & Bioactivity
EGF, Mouse, HEK293 Cells,Tag Free:Synonyms
beta-urogastrone; EGF; epidermal growth factor (beta-urogastrone); epidermal growth factor; hEGF
EGF, Mouse, HEK293 Cells,Tag Free:Background
Epidermal growth factor (EGF) is a small, potent growth factor capable of inducing cell proliferation, differentiation, and survival. EGF is the founding member of the EGF family that also includes TGF-alpha, amphiregulin (AR), betacellulin (BTC), epiregulin (EPR), heparin-binding EGF-like growth factor (HB-EGF), epigen, and the neuregulins (NRG)-1 through -6 (1). Members of The EGF family are characterized by a shared structural motif, the EGF-like domain, which contains three intramolecular disulfide bonds that are formed by six similarly spaced, conserved cysteine residues (2). These disulfide bonds are essential for proper protein conformation and receptor binding. All EGF family members are synthesized as type I transmembrane precursor proteins that may contain several EGF domains in the extracellular region. The mature proteins are released from the cell surface by regulated proteolysis (1). The full length EGF protein is 1207 amino acids (aa) (EGF precursor) containing nine EGF domains and nine LDLR class B repeats. However, the mature protein is much smaller, only 53 aa, and is generated by proteolytic cleavage of the EGF domain proximal to the transmembrane region (3). EGF is well conserved across mammals with mature human EGF 70% identical to mature mouse and rat EGF. Physiologically, EGF is found in various body fluids, including blood, milk, urine, saliva, seminal fluid, pancreatic juice, cerebrospinal fluid, and amniotic fluid (4). EGF is a high affinity ligand of the EGF receptor (ErbB). Four ErbB (HER) family receptor tyrosine kinases including EGFR/ErbB1, ErbB2, ErbB3 and ErbB4, mediate responses to EGF family members (5). EGF binding induces dimerization of the EGF receptor resulting in activation of the protein tyrosine kinase signaling pathway. These receptors undergo a complex pattern of ligand-induced homo- or hetero-dimerization to transduce EGF family signals (6, 7). EGF binds ErbB1 and depending on the context, induces the formation of homodimers or heterodimers containing ErbB2. Dimerization results in autophosphorylation of the receptor at specific tyrosine residues to create docking sites for a variety of signaling molecules (5, 8).
1. Harris, R.C. et al. (2003) Exp. Cell Res. 284:2.
2. Carpenter, G. and Cohen, S. (1990) J. Biol. Chem. 265:7709.
3. Gray, A. et al. (1983) Nature 303:722.
4. Carpenter, G. and Zendegui, J.G. (1986) Exp. Cell Res. 164:1.
5. Jorissen, R.N. et al. (2003) Exp. Cell Res. 284:31.
6. Gamett, D.C. et al. (1997) J. Biol. Chem. 272:12052.
7. Qian, X. et al. (1994) Proc. Natl. Acad. Sci. 91:1500.
8. Qian, X. et al. (1999) J. Biol. Chem. 274:574.